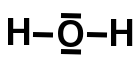Basiskennis chemie/Bindingen/Covalente binding
Covalente binding en moleculen
Atoombinding
Molecuul / Molecule
Waterstof
In de figuur hiernaast zijn de gedeelde elektronen als puntjes weergegeven tussen de twee atomen.
Verschillende atomen
Meer atomen
Meer elektronen delen
Voor stikstof geldt dat elk stikstofatoom maar vijf elektronen in zijn valentieschil heeft. Door 3 elektronen met een ander stikstof-atoom te delen ontstaat weer de edelgasconfiguratie van neon.
Meer dan 3 elektronen worden zelden met één ander atoom gedeeld.Elektronegativiteit en de polair-covalente binding
Omgekeerd geldt voor het waterstof-atoom het omgekeerde: De elektronen zijn vaker bij zuurstof, dus minder vaak bij waterstof. In de buurt van waterstof is te weinig negatieve lading aanwezig om de positieve lading van de waterstofkern te neutraliseren. In de buurt van het waterstof-atoom heeft het molecuul een positieve lading.
Om het verschil in "harder" of "minder hard" aan elektronen trekken van elementen in getallen weer te geven wordt het begrip elektronegativiteit gebruikt. In onderstaande tabel zijn de door Linus Pauling ontwikkelde waarden aangegeven.
Elektrontrekken is wel een populaire sport bij de atomen. Fluor is de ongeslagen kampioen, met zuurstof als goede tweede. De meeste metalen zijn er niet goed in. De zwaardere alkalimetalen zijn de absolute loosers.

Elektronegativiteit, waarden volgens Pauling
| || colspan="18" align="left" | Elektronegatieviteit in het periodiek systeem der elementen
|-
|width=60 style="border-style:none" |
|align=center valign=bottom width=32 style="border-style:none" | 1
Ia
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|width=32 style="border-style:none" |
|align=center valign=bottom width=32 style="border-style:none" | 18
0
|-
| align="center" | 1
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,1
H
|align=center valign=bottom style="border-style:none" | 2
IIa
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|align=center valign=bottom style="border-style:none" | 13
IIIa
|align=center valign=bottom style="border-style:none" | 14
IVa
|align=center valign=bottom style="border-style:none" | 15
Va
|align=center valign=bottom style="border-style:none" | 16
VIa
|align=center valign=bottom style="border-style:none" | 17
VIIa
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
He
|-
| align="center" | 2
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,0
Li
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
Be
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|style="border-style:none" |
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,0
B
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FFFF7D;" | 2,5
C
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FFFF7D;" | 3,1
N
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FB8605;" | 3,5
O
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FB8605;" | 4,1
F
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Ne
|-
| align="center" | 3
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,0
Na
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Mg
|align=center valign=bottom style="border-style:none" | 3
IIIb
|align=center valign=bottom style="border-style:none" | 4
IVb
|align=center valign=bottom style="border-style:none" | 5
Vb
|align=center valign=bottom style="border-style:none" | 6
VIb
|align=center valign=bottom style="border-style:none" | 7
VIIb
|align=center valign=bottom style="border-style:none" | 8
VIIIb
|align=center valign=bottom style="border-style:none" | 9
VIIIb
|align=center valign=bottom style="border-style:none" | 10
VIIIb
|align=center valign=bottom style="border-style:none" | 11
Ib
|align=center valign=bottom style="border-style:none" | 12
IIb
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
Al
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,7
Si
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,1
P
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,4
S
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FFFF7D;" | 2,8
Cl
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Ar
|-
| align="center" | 4
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 0,9
K
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,0
Ca
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Sc
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3
Ti
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
V
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,6
Cr
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,6
Mn
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,6
Fe
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,7
Co
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,8
Ni
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,8
Cu
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,7
Zn
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,8
Ga
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,0
Ge
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,2
As
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FFFF7D;" | 2,5
Se
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FFFF7D;" | 2,7
Br
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FFFF7D;" | 3,0[4]
Kr
|-
| align="center" | 5
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 0,9
Rb
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,0
Sr
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,1
Y
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Zr
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Nb
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3
Mo
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
Tc
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
Ru
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
Rh
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
Pd
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
Ag
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
Cd
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
In
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,7
Sn
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,8
Sb
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,0
Te
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,2
I
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#FFFF7D;" | 2,6[4]
Xe
|-
| align="center" | 6
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 0,9
Cs
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,0
Ba
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00ff99;" | 1,2
Hf
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3
Ta
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
W
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
Re
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
Os
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,6
Ir
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
Pt
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
Au
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
Hg
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4
Tl
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,6
Pb
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,7
Bi
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,8
Po
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,0
At
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 2,2[4]
Rn
|-
| align="center" | 7
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 0,9
Fr
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,0
Ra
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Rf
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Db
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Sg
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Bh
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Hs
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Mt
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Ds
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Rg
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Cn
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Nh
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Fl
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Mc
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Lv
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Ts
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#ffffff;" |
Og
|-
|colspan="19" style="border-style:none" |
|-
|style="border-style:none" colspan=3 | *Lanthaniden
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,1
La
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,1
Ce
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,1
Pr
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Nd
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Pm
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Sm
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,0
Eu
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,1
Gd
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Tb
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Dy
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Ho
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Er
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Tm
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,1
Yb
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,2
Lu
|-
|style="border-style:none" colspan=3 | **Actiniden
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,0
Ac
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3
Th
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,5
Pa
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#33CC33;" | 1,7
U
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,4[4]
Np
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
Pu
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,1[4]
Am
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
Cm
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
Bk
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
Cf
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
Es
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
Fm
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
Md
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
No
|align=center valign=bottom style="border-style:solid; border-width:1px; border-color:#888888; background:#00FF99;" | 1,3[4]
Lr
|}
| < 1.7 | 1.7 - 2.4 | 2.5 -3.2 | > 3.3 |
Een voorbeeld waarbij ΔEN nul is, is zuurstof O2. De covalente binding tussen niet-identieke atomen is in principe polair, het ene atoom is een beetje positief, het andere een beetje negatief. De sterkte van de polariteit hangt af van het verschil in elektronegativiteit tussen beide atomen. Covalente bindingen met ΔEN waarden kleiner dan 0,4 hebben in de meeste gevallen een apolair karakter. Atoombindingen met ΔEN waarden tussen 0,4 en 1,7 zijn in de meeste gevallen polair. Een voorbeeld hiervan is de sterk polaire O-H-binding met ΔEN = 1,4 die men in water en alcoholen aantreft.
|- ! align="center" | ΔEN !! Soort binding |- | < 0,4 || Covalente binding |- | 0,4 < 1,7 || Polair covalente binding |- | > 1,7 || Ionbinding
|}Polair covalent
Ionogeen
Notatie
- ↑ De bananenbinding wordt hier bewust buiten beschouwing gelaten.
- ↑ In het Noord-Nederlands is het woord "Molecuul" onzijdig: het molecuul. Er wordt naar verwezen met woorden als dit, dat en zijn. In het Zuid-Nederlands (Vlaams) wordt gesproken over de molecule, deze, die en haar.
- ↑ De exacte grootte van het percentage is niet echt belangrijk, maar maakt de manier van redeneren hier wel makkelijker.
- ↑ 4,00 4,01 4,02 4,03 4,04 4,05 4,06 4,07 4,08 4,09 4,10 4,11 4,12 4,13 https://commons.wikimedia.org/wiki/File:Electronegative.jpg (20191125 1200)